What we’re working on…
Synaptic transmission & heterogeneity
Synapses of different maturity and constitution coexist at the Drosophila NMJ, giving us the opportunity to investigate the influence of synaptic heterogeneity on neurotransmission. We use genetics, live-imaging, pharmacology and electrophysiology to do this.
To find how components of the presynapse influence synaptic transmission, we investigate single proteins of interest regarding their functional domains and their control of spontaneous and action potential evoked vesicle exocytosis. Synaptic proteins like Unc13 can interact with lipids and other proteins involved in the plastic adaptation of synaptic strength. Therefore, we use mutant animals, electrophysiology and pharmacology to interfere with and measure synaptic strength.
Synaptopathies
Synaptopathy is an umbrella term used for diseases affecting synaptic transmission. Our colleagues from Hacettepe University have identified patients that harbor new mutations causing inherited neurometabolic diseases putatively affecting synaptic transmission. Our aim is to find out if the candidate gene is linked to synaptopathy, and also to dissect the cellular pathways that are responsible for the abnormal function by using Drosophila as a model organism.
Synaptic Plasticity
Synaptic transmission is not a static process and can change over time in response to activity and external cues. This modulation of neurotransmission is referred to as Synaptic Plasticity and forms the basis of learning, memory, information processing, and sensory adaptation.
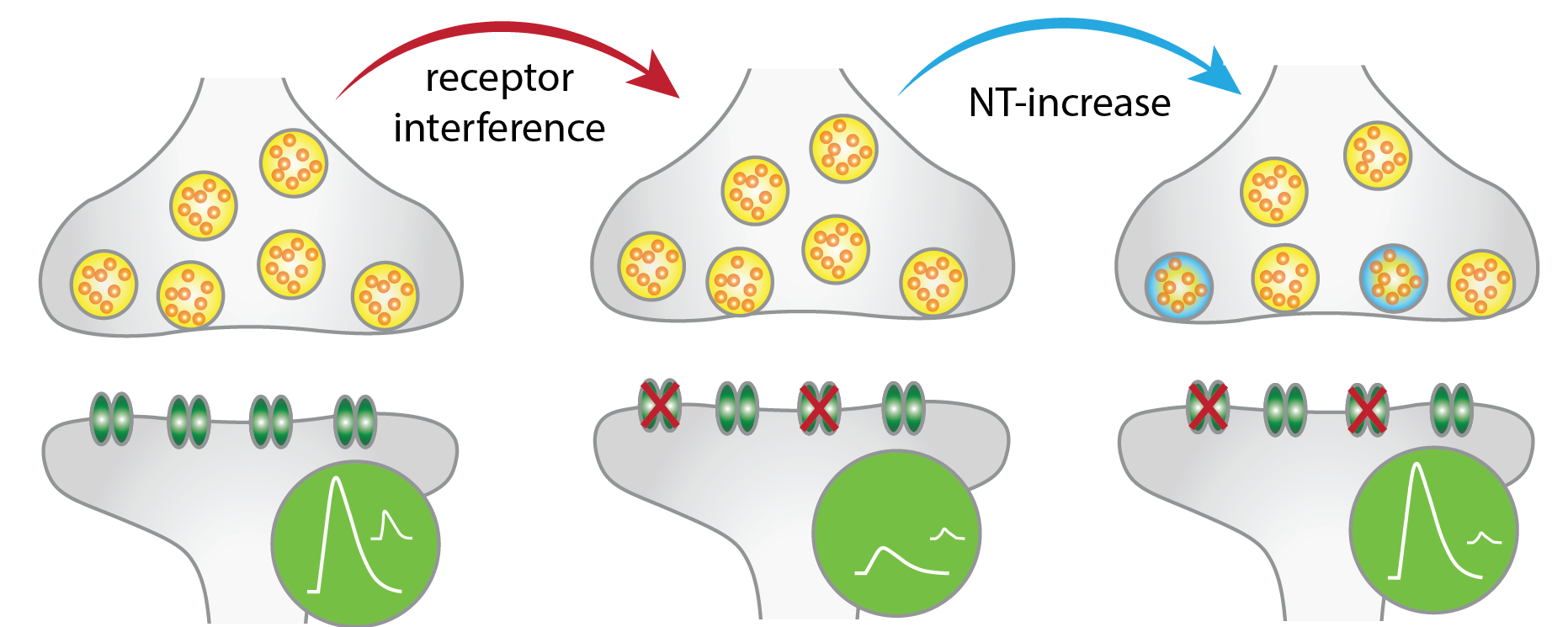
Homeostatic plasticity maintains normal neurotransmission in the face of synaptic challenge. We study the presynaptic structural reorganisation of proteins and functional changes that occur as a result of postsynaptic perturbation.
Short-term plasticity describes the use-dependent strengthening or weakening of neurotransmission on the timescale of milliseconds to minutes. We aim to understand the role the of presynaptic components in this process.
Mathematical Modeling and Simulations
Complex processes are at play to ensure proper communication between neuronal cells at the synapse. To help us understand how certain molecules direct this so that synapses can work normally, we use the power of modern computers. This is one of the main pillars of our work.
Mathematical equations can be used to describe what we assume happens in the cell. Feeding these equations (the “model”) into the appropriate software allows the computer to evaluate our assumptions much faster than any human could. In a back and forth comparison between the experimental data and these simulations, we optimize certain details of the model. If our assumptions are close to reality, and if it is not overly complex, the model will make meaningful predictions which can be tested in other experiments. This feedback loop between experimental and theoretical work constitutes a powerful tool to investigate neurotransmission.